
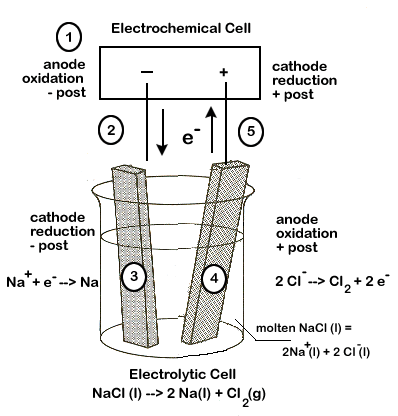
The electrolysis cell consists of two metallic rods known as electrodes that are connected to a battery and dipped into molten electrolyte or electrolyte solution.At 25 ☌ with pH 7 (H + 1.0 × 10 7 M), the potential is unchanged based on the Nernst equation.
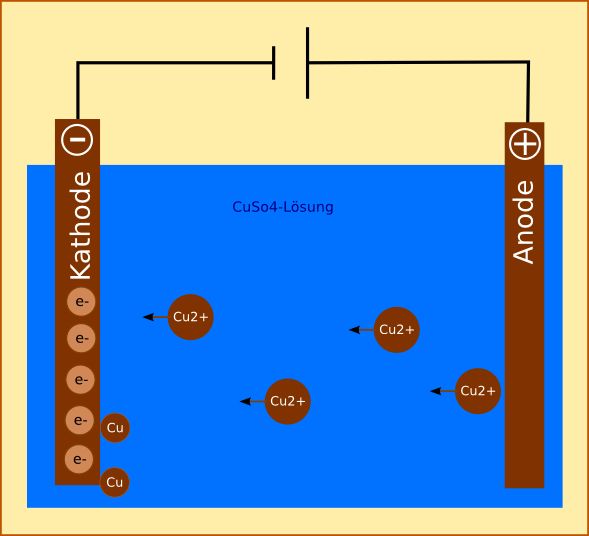
Electrolysis is a process by which electrical energy is used to produce a chemical change. Thus, the standard potential of the water electrolysis cell (E o cell E o cathode E o anode) is 1.229 V at 25 ☌ at pH 0 (H + 1.0 M).JEE Main 2022 Question Paper Live Discussion.Difference Between Selling And Marketing.TS Grewal Solutions Class 11 Accountancy.TS Grewal Solutions Class 12 Accountancy.
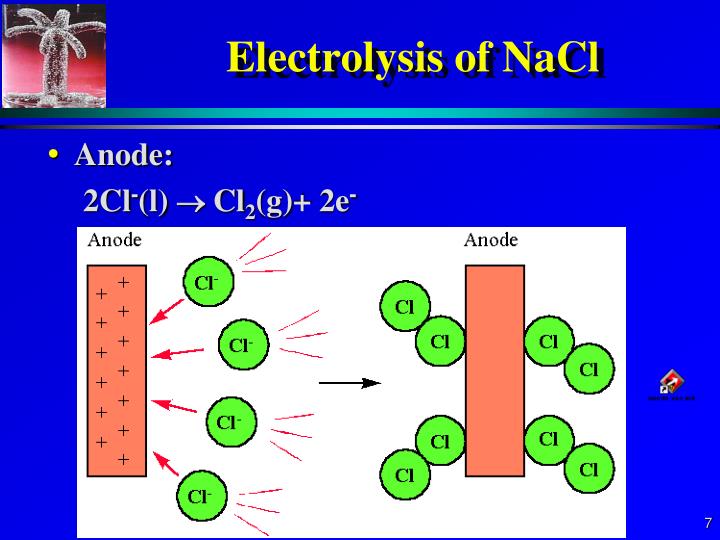
#Cathode reaction in electrolytsis of cucl2 free#
Calculate the grams of metal or liters of gas fo At which electrode is the free metal produced in the electrolysis of a metal compound Why What half-reaction would be expected to occur at the cathode in the electrolysis of aqueous. CBSE Previous Year Question Papers Class 12 Predict the products of the electrolysis and write the reactions occurring at the anode and cathode. Partial electrolysis experiments were also used to confirm the formation of CaTiO3 in molten CaCl2, particularly short-time electrolysis (1, 5, 10, 20, and 30 minutes).CBSE Previous Year Question Papers Class 10.NCERT Solutions For Class 6 Social Science.NCERT Solutions for Class 7 Social Science.NCERT Solutions for Class 8 Social Science.


 0 kommentar(er)
0 kommentar(er)
